Carbon Dating Undercuts Evolution's Long Ages
by John Baumgardner, Ph.D.
...With the discovery of radioactivity about a hundred years ago, evolutionists deeply committed to the uniformitarian outlook believed they finally had proof of the immense antiquity of the earth. In particular, they discovered the very slow nuclear decay rates of elements like Uranium while observing considerable amounts of the daughter products from such decay. They interpreted these discoveries as vindicating both uniformitarianism and evolution, which led to the domination of these beliefs in academic circles around the world throughout the twentieth century.
However, modern technology has produced a major fly in that uniformitarian ointment. A key technical advance, which occurred about 25 years ago, involved the ability to measure the ratio of 14C atoms to 12C atoms with extreme precision in very small samples of carbon, using an ion beam accelerator and a mass spectrometer. Prior to the advent of this accelerator mass spectrometer (AMS) method, the 14C/12C ratio was measured by counting the number of 14C decays. This earlier method was subject to considerable "noise" from cosmic rays.
The AMS method improved the sensitivity of the raw measurement of the 14C/12C ratio from approximately 1% of the modern value to about 0.001%, extending the theoretical range of sensitivity from about 40,000 years to about 90,000 years. The expectation was that this improvement in precision would make it possible to use this technique to date dramatically older fossil material.1 The big surprise, however, was that no fossil material could be found anywhere that had as little as 0.001% of the modern value!2 Since most of the scientists involved assumed the standard geological time scale was correct, the obvious explanation for the 14C they were detecting in their samples was contamination from some source of modern carbon with its high level of 14C. Therefore they mounted a major campaign to discover and eliminate the sources of such contamination. Although they identified and corrected a few relatively minor sources of 14C contamination, there still remained a significant level of 14C—typically about 100 times the ultimate sensitivity of the instrument—in samples that should have been utterly "14C-dead," including many from the deeper levels of the fossil-bearing part of the geological record.2
Let us consider what the AMS measurements imply from a quantitative standpoint. The ratio of 14C atoms to 12C atoms decreases by a factor of 2 every 5730 years. After 20 half-lives or 114,700 years (assuming hypothetically that earth history goes back that far), the 14C/12C ratio is decreased by a factor of 220, or about 1,000,000. After 1.5 million years, the ratio is diminished by a factor of 21500000/5730, or about 1079. This means that if one started with an amount of pure 14C equal to the mass of the entire observable universe, after 1.5 million years there should not be a single atom of 14C remaining! Routinely finding 14C/12C ratios on the order of 0.1-0.5% of the modern value—a hundred times or more above the AMS detection threshold—in samples supposedly tens to hundreds of millions of years old is therefore a huge anomaly for the uniformitarian framework.
This earnest effort to understand this "contamination problem" therefore generated scores of peer-reviewed papers in the standard radiocarbon literature during the last 20 years.2 Most of these papers acknowledge that most of the 14C in the samples studied appear to be intrinsic to the samples themselves, and they usually offer no explanation for its origin. The reality of significant levels of 14C in a wide variety of fossil sources from throughout the geological record has thus been established in the secular scientific literature by scientists who assume the standard geological time scale is valid and have no special desire for this result!
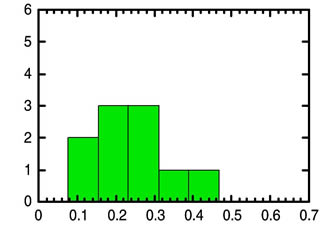
In view of the profound significance of these AMS 14C measurements, the ICR Radioisotopes and the Age of the Earth (RATE) team has undertaken its own AMS 14C analyses of such fossil material.2 The first set of samples consisted of ten coals obtained from the U. S. Department of Energy Coal Sample Bank maintained at the Pennsylvania State University. The ten samples include three coals from the Eocene part of the geological record, three from the Cretaceous, and four from the Pennsylvanian. These samples were analyzed by one of the foremost AMS laboratories in the world. Figure 1 below shows in histogram form the results of these analyses.
Percent Modern Carbon
These values fall squarely within the range already established in the peer-reviewed radiocarbon literature. When we average our results over each geological interval, we obtain remarkably similar values of 0.26 percent modern carbon (pmc) for Eocene, 0.21 pmc for Cretaceous, and 0.27 pmc for Pennsylvanian. Although the number of samples is small, we observe little difference in 14C level as a function of position in the geological record...
...Applying the uniformitarian approach of extrapolating 14C decay into the indefinite past translates the measured 14C/12C ratios into ages that are on the order of 50,000 years (2-50000/5730 = 0.0024 = 0.24 pmc)...
...If one takes as a rough estimate for the total 14C in the biosphere before the cataclysm as 40% of what exists today and assumes a relatively uniform 14C level throughout the pre-Flood atmosphere and biomass, then we might expect a 14C/12C ratio of about 0.4% of today's value...
Some readers at this point may be asking, how does one then account for the tens of millions and hundreds of millions of years that other radioisotope methods yield for the fossil record? Most of the other RATE projects address this important issue. Equally as persuasive as the 14C data is evidence from RATE measurements of the diffusion rate of Helium in zircon crystals that demonstrates the rate of nuclear decay of Uranium into Lead and Helium has been dramatically higher in the past and the uniformitarian assumption of a constant rate of decay is wrong.3 Another RATE project documents the existence of abundant Polonium radiohalos in granitic rocks that crystallized during the Flood and further demonstrates that the uniformitarian assumption of constant decay rates is incorrect.4 Another RATE project provides clues for why the 14C decay rate apparently was minimally affected during episodes of rapid decay of isotopes with long half-lives.5
http://www.icr.org/article/117/
